

In all sapphire cases, the surface was polished using a 0.10μm alumina slurry. This was followed by careful rinsing and inspection. The surface was accepted when the water contact angle was zero.
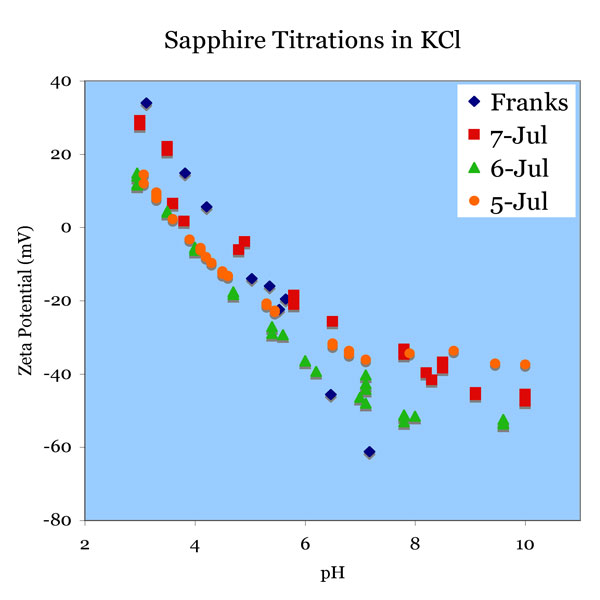
July 5th. A polished sapphire sample was pre-treated in pH 11 solution (30 minutes)and used in 1mM KCl solution. The solution was titrated from pH 10 to pH 3.5.
July 6th. A sapphire sample was sonicated after polishing. It was then pre-treated in pH 11 solution (30 minutes) and used in 1 mM KCl solution. The solution was titrated from pH 10 to pH 3.5.
July 7th. A sapphire sample was sonicated after polishing. It was then pre-treated in pH 11 solution (30 minutes) and used in 1 mM KCl solution. The solution was titrated from pH 10 to pH 3.5.
The purpose of sonication was to remove remaining alumina particles. Sonication was retained as a step in pre-treatment, but it had no or minimal effect on the titration data. Note that the isoelectric point is around a pH of 4, while for the mica surface, which has a silicate surface unless aluminum species have been dissolved at low pH and re-deposited, the isoelectric point is below 3. Comparative data can be found in [Franks03].
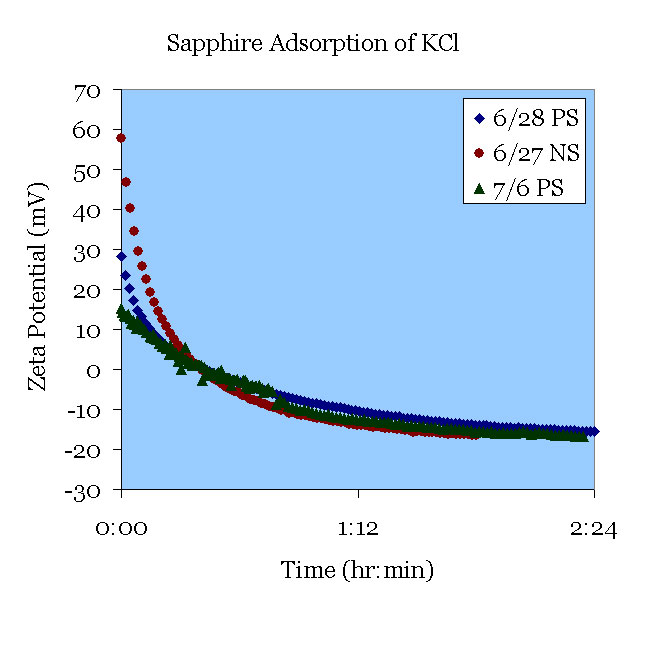
June 27th. A polished sapphire sample was used in 160 μS/cm KCl solution. The aging of KCl was observed at pH 5.5.
June 28th. A polished sapphire sample was pre-treated in pH 11 solution and used in 138 μS/cm KCl solution. The aging of sapphire in KCl was observed at pH 5.5.
July 6th. A polished sapphire sample was sonicated. It was then pre-treated in pH 11 solution and used in 148 μS/cm KCl solution. The aging of sapphire in KCl was observed at pH 5.5. All plots proved to be reproducible over time.